Veeva Creator Series interview with Mark Johnson: Providing the 360-Degree View Customers Have Always Wanted
The Veeva Creator Series is a regularly published series of interviews with Veeva product leaders and strategists highlighting the backstories to key CRM innovations. This month, we interview Mark Johnson, VP of Product Management, about the Veeva CRM timeline view.
What is the timeline view?
The timeline view is unique way to visualize healthcare provider (HCP) interactions over a given period of time, be it monthly, quarterly or annually. The beauty of the timeline is not only that you can you see all of your interactions in an intuitive manner, but you also more easily see what is NOT happening. Visualizations allow you to fill in those critical gaps that can make all the difference. So, timeline is designed to be a ‘consume-first’ interface rather than your traditional data entry screen that most CRM systems have because it’s what reps really need when they prepare for a call.
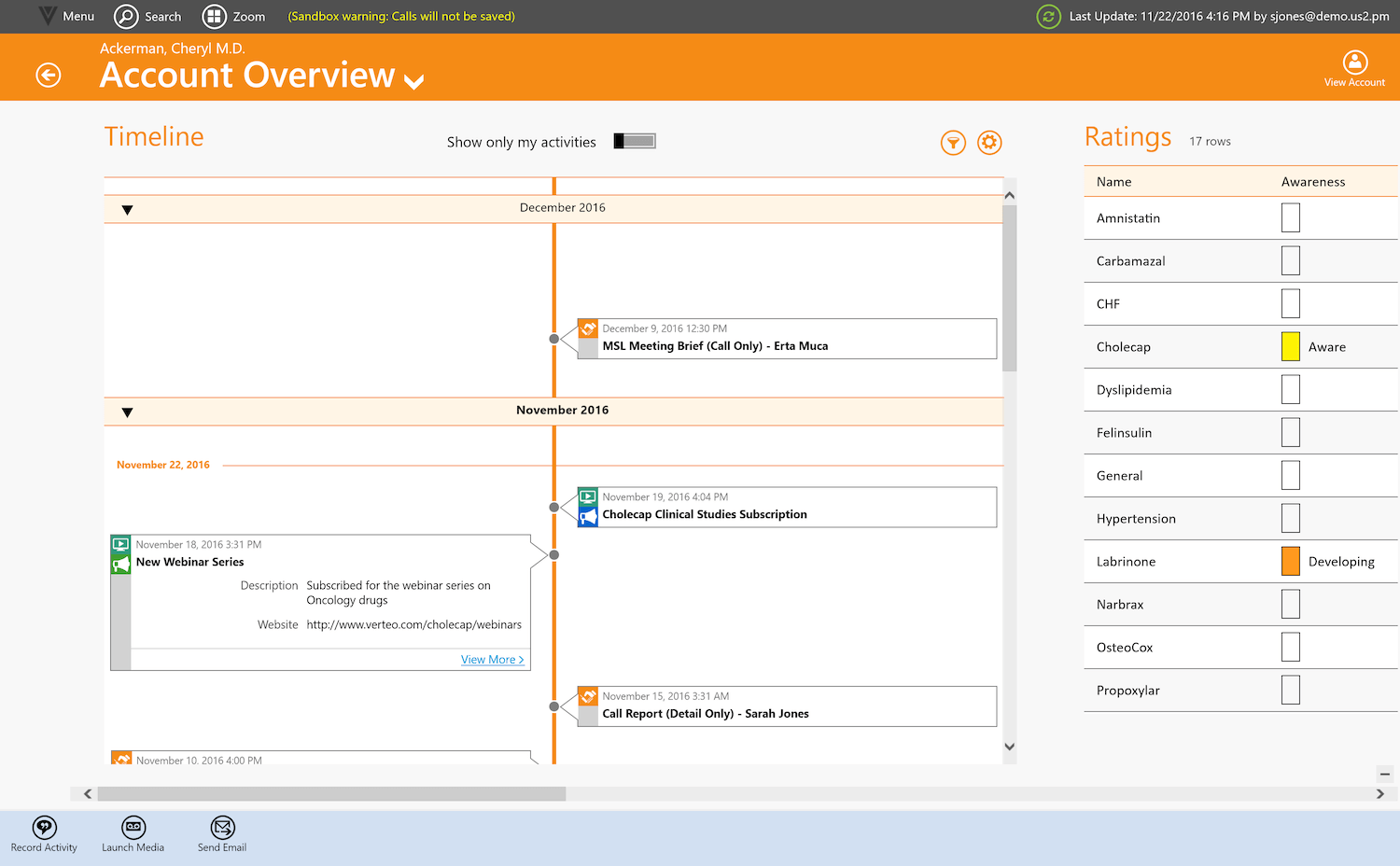
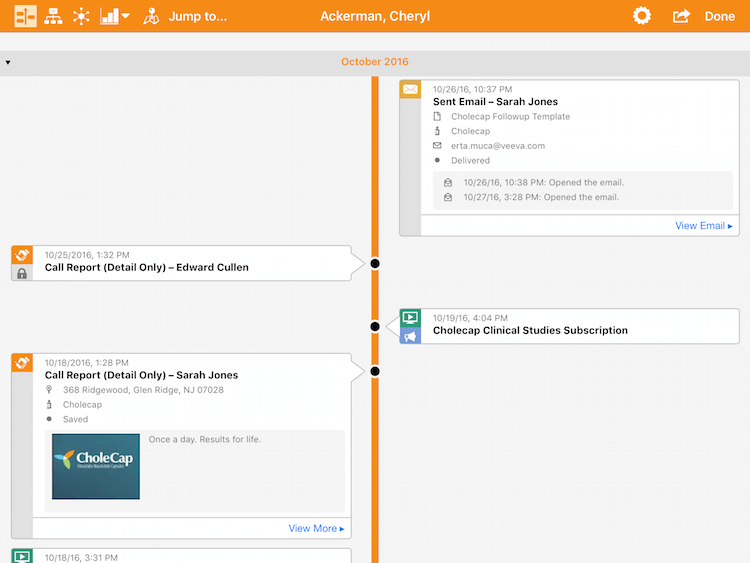
Timeline view from Windows device
This concept ties back directly to customer relationship management, which is fundamentally about understanding customers. For CRMs to be effective, reps need access to as much quality information as possible. But just because we can list information doesn’t mean it is consumable for them. Surfacing it in a graphical manner helps address the challenge.
It makes logical sense, if you think about it. After all, the human brain is graphically wired; we see everything in images rather than text. Just think of a child or parent, for instance. It’s not their name that sprang to mind but their image. That is simply how we process information. So, it generally makes sense to display data in a visual manner because it is much more intuitive. This concept is common in social media; Facebook and LinkedIn have already figured this out. At Veeva, we’re the first ones to “consumerize” this type of thinking into the pharma space.
What was the genesis for the timeline?
The timeline view is an example of Veeva’s investment and emphasis on innovation. Customers didn’t overtly come to us and say, “we want a chronological timeline view.” But when we went on rep ride-alongs, we did hear them ask how they could better understand their next interaction. So it was a realization we had out in the field.
There’s a common refrain among customers that they desire a “360-degree view” of the HCP. What they’re really saying is, how can we get the entire organization on the same page? They simply can’t have one rep stop by to drop off samples one week while another rep does the same a week later. With timeline, we recognized that part of the value would be to help the company coordinate better.
What was the biggest challenge in developing timeline?
Without question, building the right user interface (UI) was the most complex aspect of it. We knew we had to find the balance between delivering the right and the sufficient amount of information. We could have surfaced lots of data from related lists, but it’s simply too much to show. We needed to focus on the valuable information and display it in an intuitive manner. Since the tablet user generally tends to scroll in an ‘up/down’ manner, we let that be our guide.
The problem was that the UI needed to retain its scale – it was a problematic task. We came up with an approach in which we highlighted the thumb nail of the interaction first, and then let the user drill down. Approved Email is a good example. It shows the basic information at a glance, but can be expanded upon for further detail.
What is your big takeaway about the timeline view?
Certainly, we want to think of it as the first place that a rep should be going to. If there’s a scheduled call, they should come to the timeline to review what has been done and what remains outstanding. In the timeline view, the rep can easily see the basic info they need to be successful, such as product metrics, formulary status, etc. The way reps interact with CRM needs to evolve and timeline offers that capability.
The functionality is relatively new and adoption is growing. Customers who are using it understand the value. People are already asking for marketing integration, which tells us that the they’re thinking about how to leverage its full potential. Some have even gone further and inquired about integration of medical information as well. One customer actually commented that “this is the 360-degree view we’ve always wanted.” It’s exciting to have that kind of feedback.
You can see the timeline in action by viewing the Veeva CRM demo video.
About Mark
Mark Johnson is responsible for the Veeva CRM roadmap, product innovation and customer success. He has been with Veeva for over two years and comes from a background of both product management and software engineering. His favorite thing about Veeva is the speed of solution delivery: “Our product roadmap is always ambitious. I love to watch the team execute on it, to make those innovations real for our customers. It’s a constant source of pride for us.”



 Accurate customer data is essential to increasing sales and marketing efficiency, improving analysis and decision-making, and gaining a complete, real-time view of the customer. When the right customer data flows into a CRM system, sales teams can drive meaningful interactions with HCPs and stay ahead on compliance efforts. For example, accurate specialty data in CRM helps sales reps avoid the potential for off-label detailing.
Accurate customer data is essential to increasing sales and marketing efficiency, improving analysis and decision-making, and gaining a complete, real-time view of the customer. When the right customer data flows into a CRM system, sales teams can drive meaningful interactions with HCPs and stay ahead on compliance efforts. For example, accurate specialty data in CRM helps sales reps avoid the potential for off-label detailing.