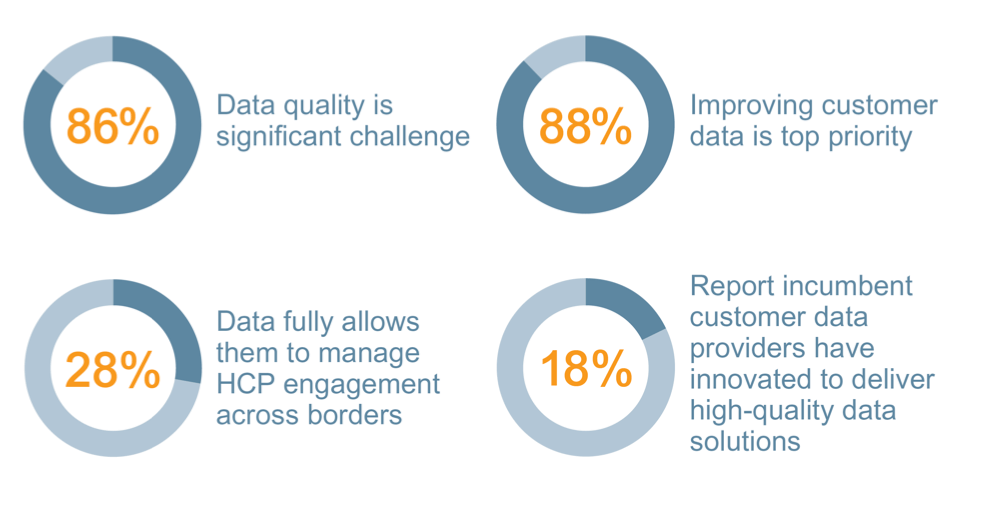
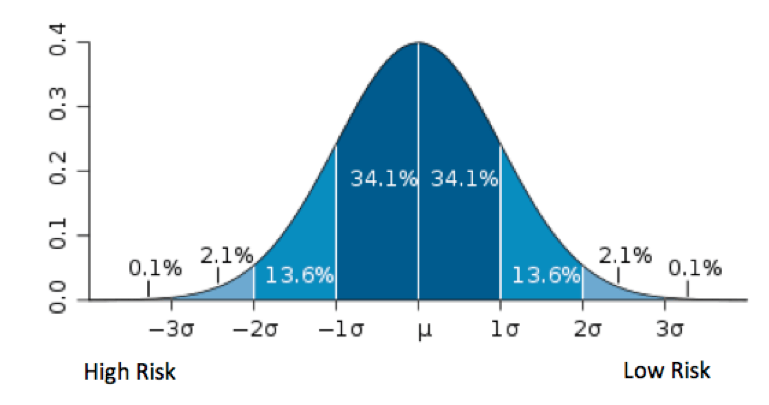
The pharmaceutical industry has a reputation for making significant investments in risk management. After all, they manage a business with a high level of risk – financial risk from very expensive research programs, reputational risk from testing new compounds on humans, and constant regulatory compliance risk. Mitigating these risks are a core determinant of success, and a quality risk management (QRM) approach that is well understood and commonly accepted is required.
I recently had the opportunity to present at the Institute of Validation Technology (IVT) Quality Risk Management conference, which brought together quality professionals from pharmaceutical companies. One of the hot topics was the industry’s true level of maturity in applying QRM principles. Recent press about poor product quality suggests pharmaceutical companies may not be far along the ‘Risk Maturity Model1‘, and there are many opportunities for improvement.
We explored recent research in QRM2, and trends in compliance enforcement actions – namely FDA warning letters:
- Drug product recalls from 2006-2013 is increasing
- Recalls related to product quality is increasing
- cGMP-related warning letters with 1 or more QRM citations is increasing
- The majority of QRM citations issued were due to a failure to apply QRM
In looking at the five phases of the Risk Maturity Model, these findings indicate that some pharmaceutical companies are between Phase 1 and Phase 2, where QRM activities are informal, inconsistent, and insufficient.

While not a reflection of QRM adoption in every company, it’s nonetheless a good call to action as quality professionals expand efforts to improve product quality.
To support continuous improvement in quality, please join a quality roundtable with thought leaders on Thursday, May 28, 2015 to discuss how organizations can move up the quality maturity curve with risk management being a key aspect. Look for additional information in the coming weeks.
1. Risk Maturity Model adapted from PDA Technical Report No. 54. PDA Technical Report No. 54. “Implementation of Quality Risk Management for Pharmaceutical and Biotechnology Manufacturing Operations.” 2012.
2. Quality Risk Management: Diagnosis or Cure?, Kelly Waldron, PhD Researcher, January 25, 2015


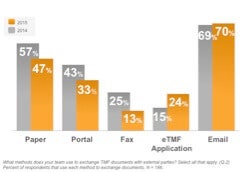
 Sponsor-CRO collaboration and the large number of documents managed by clinical operations departments (typically more than half of all TMF documents) are obvious pain points for the industry to start migrating into electronic processes. It was not surprising then to see this drop in paper in clinops and a jump in the use of eTMF applications among sponsors and CROs. Likewise, the fact that almost 60% of respondents electronically archive documents is in line with this industry transformation.
Sponsor-CRO collaboration and the large number of documents managed by clinical operations departments (typically more than half of all TMF documents) are obvious pain points for the industry to start migrating into electronic processes. It was not surprising then to see this drop in paper in clinops and a jump in the use of eTMF applications among sponsors and CROs. Likewise, the fact that almost 60% of respondents electronically archive documents is in line with this industry transformation.
 Another important finding is respondents extensively using TMF data see significantly greater benefits, particularly improved inspection readiness and cost savings. This should be a clarion call for the industry to evaluate performance metrics as part of any ROI analysis when selecting eTMF systems.
Another important finding is respondents extensively using TMF data see significantly greater benefits, particularly improved inspection readiness and cost savings. This should be a clarion call for the industry to evaluate performance metrics as part of any ROI analysis when selecting eTMF systems.